RevOps Engineered for Pharmaceutical Manufacturing
Navigate complex regulatory environments, multi-layer buying committees, and 12-18 month sales cycles with revenue operations built specifically for pharma.
Why Pharma Revenue Teams Struggle
These are the operational bottlenecks we solve with structured RevOps for pharmaceutical manufacturing businesses.
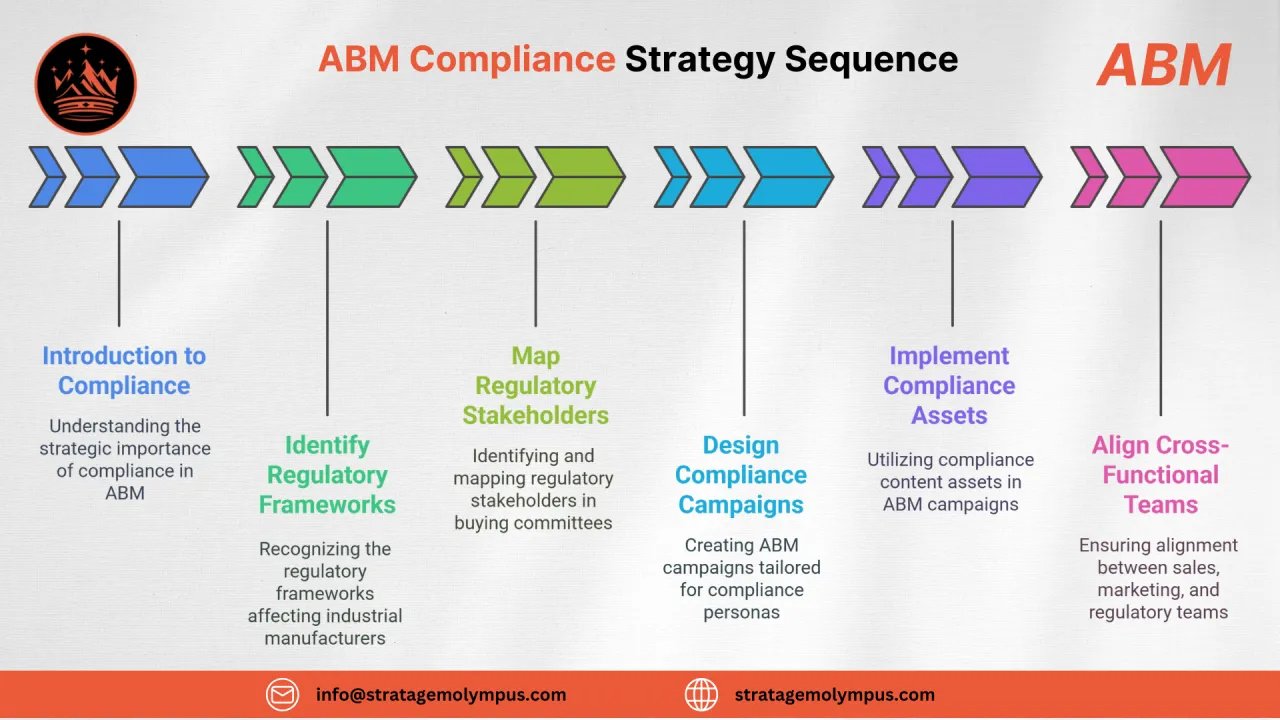
Regulatory-Driven Buying Inertia 01
GMP, FDA 21 CFR Part 11, and EU Annex 11 compliance add validation layers that stall procurement. Marketing and sales teams must address regulatory concerns proactively to prevent deals from dying in committee.
Fragmented Buying Committees 02
Pharma purchases involve QA/QC, engineering, procurement, regulatory affairs, and C-suite. Each stakeholder has distinct evaluation criteria, making it nearly impossible to win without targeted multi-persona content.
Proof-of-Concept Bottlenecks 03
Prospects demand extensive validation data, site audits, and reference installations before signing. Without a structured enablement process, these proof-of-concept stages extend timelines by months.
Siloed Sales & Marketing Data 04
CRM records rarely capture regulatory context, validation status, or compliance milestones. Sales and marketing work from incomplete data, missing signals that indicate where a deal truly sits.
Content That Doesn't Speak Regulatory 05
Generic B2B collateral fails to address cGMP requirements, cleanroom classifications, or batch record integration. Pharma buyers disengage when content shows no understanding of their compliance reality.
Who's in the Pharma Buying Committee
VP Engineering / Plant Manager
Production uptime, integration with existing lines, validation timeline
Technical specs, integration case studies, project timelines
Quality Assurance Director
GMP compliance, 21 CFR Part 11, audit readiness
Validation documentation, compliance whitepapers, regulatory checklists
Procurement / Supply Chain Lead
Total cost of ownership, vendor qualification, lead times
ROI calculators, vendor comparison guides, SLA templates
Regulatory Affairs
Change control impact, submission timelines, agency feedback risk
Regulatory impact assessments, pre-submission guidance docs
CFO / Finance
CapEx justification, payback period, budget phasing
Financial models, TCO analysis, financing options
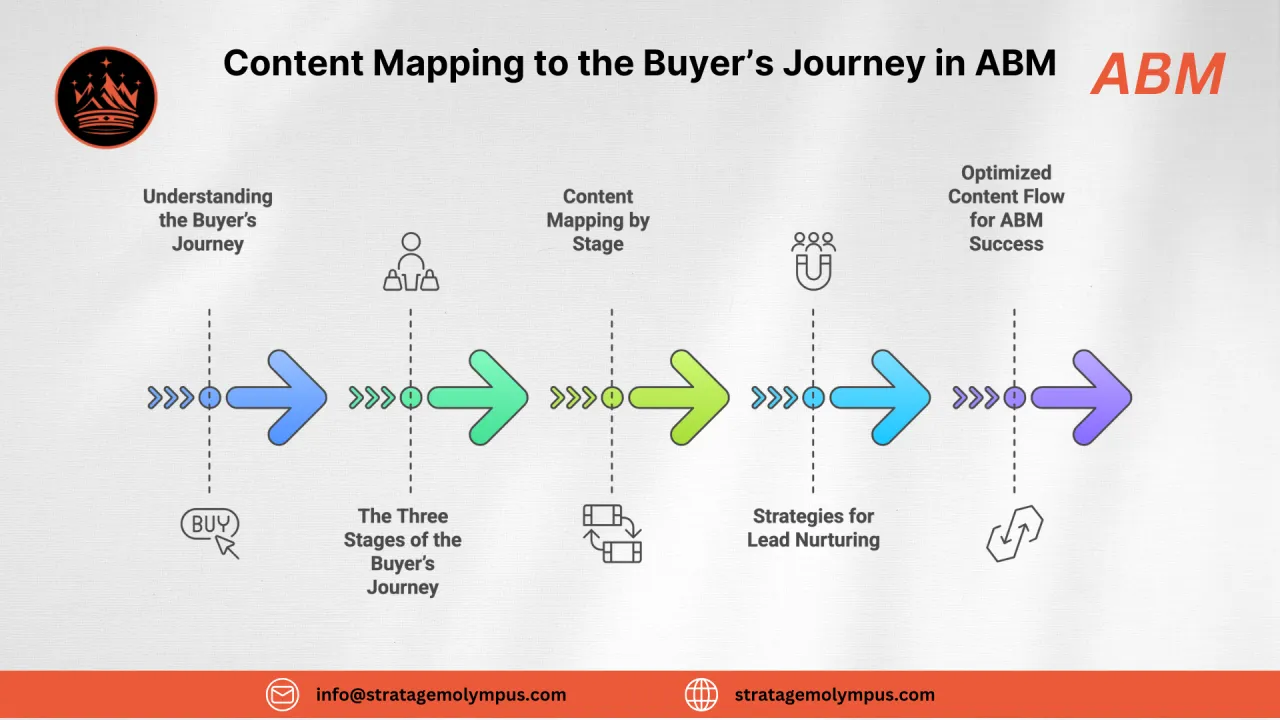
The Pharma Sales Cycle
Pharma sales cycles are extended by validation protocols, regulatory reviews, and multi-site approval processes. Deals typically pass through technical evaluation, compliance validation, and financial approval before PO.
Discovery & Qualification
Identify regulatory requirements, production gaps, and budget authority
Technical Evaluation
On-site assessments, spec matching, integration feasibility studies
Compliance & Validation
GMP documentation review, IQ/OQ/PQ planning, regulatory impact analysis
Commercial Negotiation
TCO modeling, SLA definition, procurement terms alignment
Executive Approval & PO
Final committee sign-off, CapEx approval, contract execution
SOHQ Services Mapped to Pharma
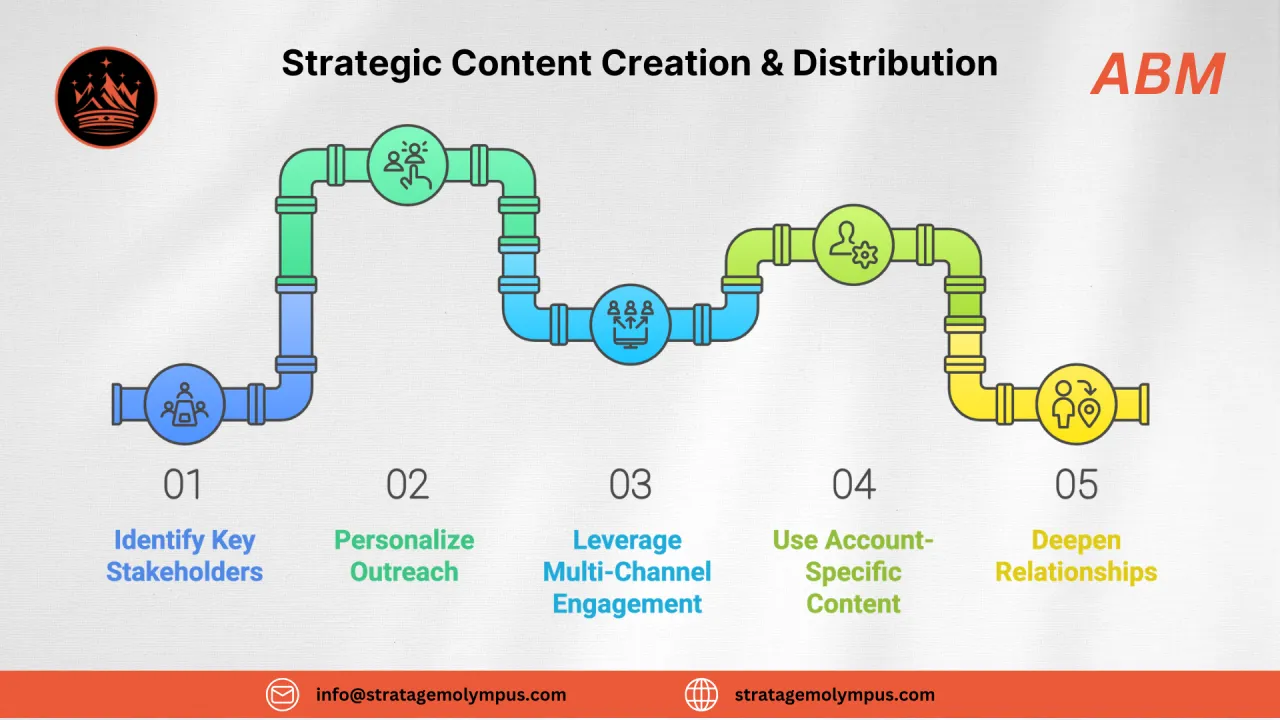
Account-Based Marketing
Target pharma buying committees with compliance-aware campaigns that engage QA, engineering, and procurement simultaneously
B2B Demand Generation
Publish regulatory-focused thought leadership (cGMP guides, validation frameworks) that attracts qualified pharma prospects
Sales & Buyer Enablement
Arm sales with validation docs, compliance checklists, and ROI models that address pharma-specific objections
Custom AI Agents + Workflows
Automate lead scoring based on regulatory signals, track validation milestones, and trigger stage-appropriate content

Unlock Efficiency, Drive Growth, Achieve Excellence Together
Are you ready to revolutionize your business operations and drive sustainable growth? Partner with us for innovative RevOps solutions tailored to your unique needs and goals.
Let's Work Together