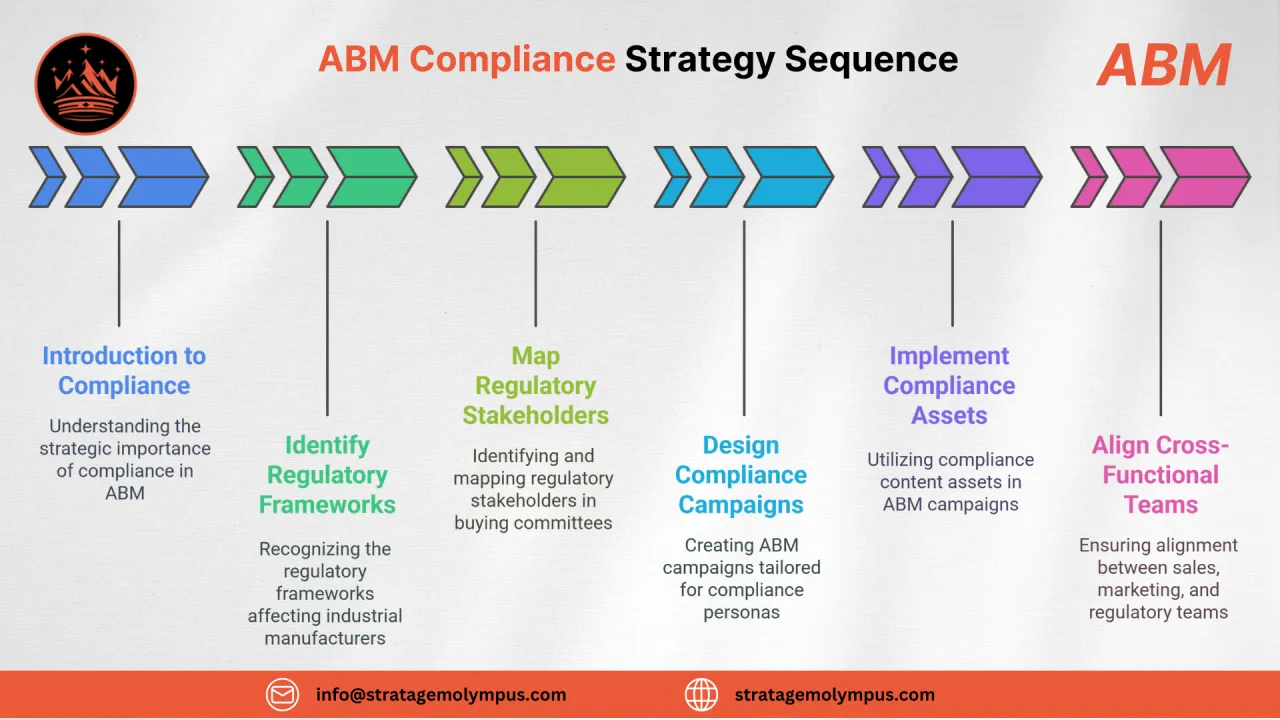
Why Compliance is a Strategic Lever in ABM?
In industrial manufacturing, compliance isn’t just a checkbox, it’s a qualification filter, a buying trigger, and a deal accelerator.
For Account-Based Marketing (ABM) to be truly effective in regulated industrial sectors, be it food processing, medical devices, pharmaceuticals, or chemical engineering, it must speak to the auditors, not just the buyers.
🏭 Traditional ABM Mistake?
Focusing only on engineers and procurement leads while completely ignoring the Regulatory Affairs, QA/QC, and Compliance personas that hold significant sway over vendor selection and purchase approval.
Whether it’s FDA’s FSMA requirements for food equipment, 21 CFR Part 11 for pharma tech, RoHS/REACH regulations for electronics, or ISO certifications across industries, compliance influences who makes it to the shortlist.
📌 According to a report by Accenture, 78% of B2B buyers in regulated industries say regulatory readiness and documentation influence their vendor selection [Source: Accenture B2B Customer Experience Study, 2022].
🤝 ABM Without Compliance? You’re Not Even in the Conversation.
An ABM strategy that doesn’t factor in regulatory touchpoints can:
- Miss key stakeholders in the buying committee
- Fail to qualify for vendor onboarding
- Undermine trust before the first sales call
On the flip side, ABM campaigns built with regulatory nuance are trusted faster, shared internally, and often become embedded into the buyer’s audit and evaluation process.
In this blog, we’ll unpack how industrial marketers can:
- Align ABM with sector-specific regulatory demands
- Map and engage compliance-related decision-makers
- Build content and messaging that satisfies both commercial and compliance criteria
- Securely deliver high-sensitivity documents via gated, trackable channels
This is not just about playing by the rules, it’s about turning regulatory literacy into competitive advantage.
Common Regulatory Frameworks Impacting Industrial Manufacturers
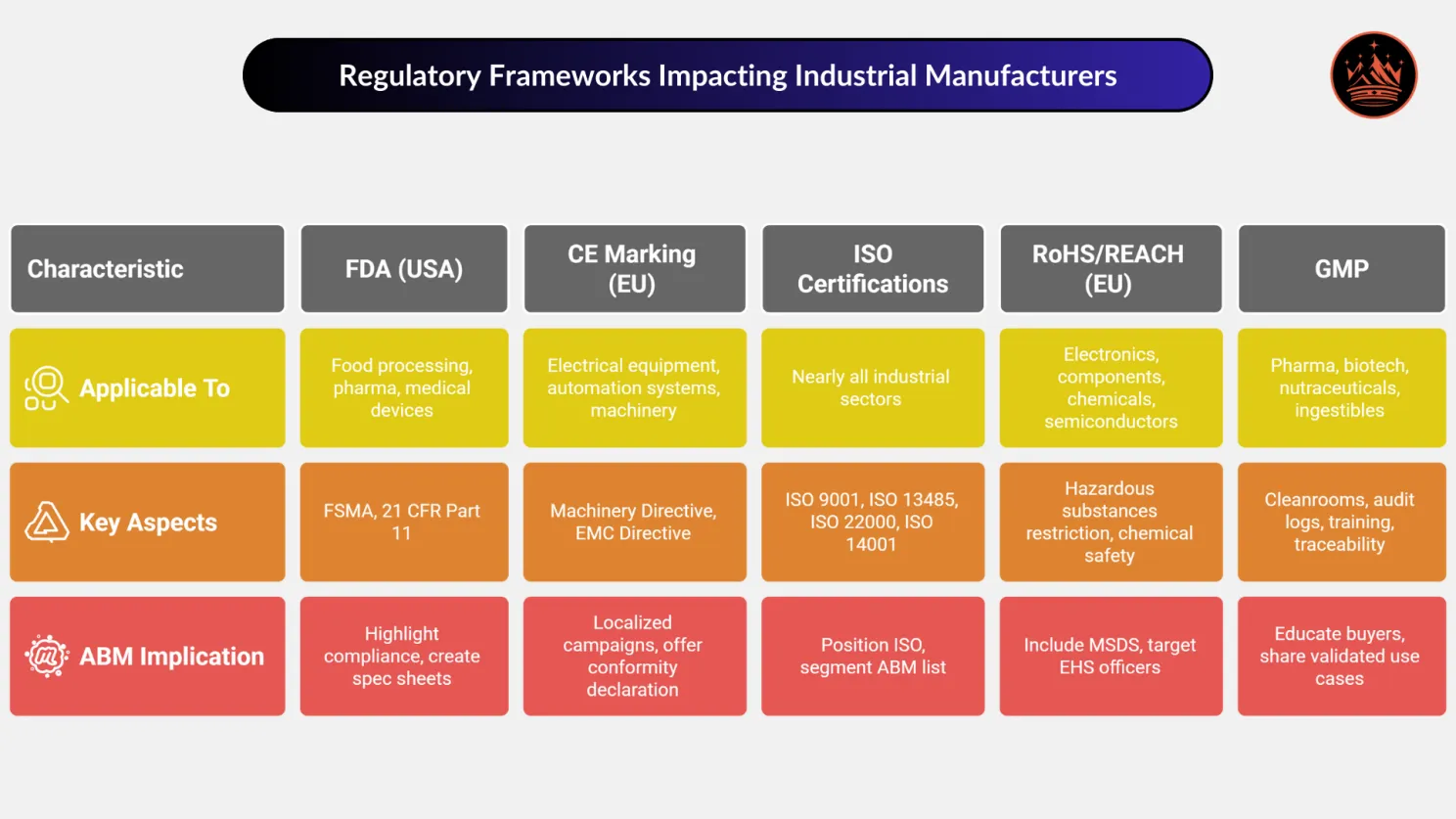
Regulatory frameworks vary across sectors, geographies, and product categories, but they all influence how vendors are evaluated, approved, and retained. For ABM to perform in regulated markets, it must anticipate what compliance officers care about long before the RFP lands.
Here’s a breakdown of core compliance frameworks shaping ABM strategy in industrial manufacturing:
📜 FDA - Food & Drug Administration (USA)
Applicable To: Food processing equipment, packaging machinery, pharma manufacturing, medical devices.
Key Regulations: FSMA (Food Safety Modernization Act) - Impacts sanitary design, cleaning protocols, documentation. 21 CFR Part 11 - Governs digital record-keeping and electronic signatures in pharma.
ABM Implication: Highlight equipment’s compliance features upfront. Create downloadable spec sheets for sanitation and validation. Include QA/QC and Compliance heads in buyer personas.
🇪🇺 CE Marking - European Conformity Standards
Applicable To: Electrical equipment, automation systems, industrial machinery sold within the EU.
Key Directives: Machinery Directive (2006/42/EC) EMC Directive (2014/30/EU)
ABM Implication: Create localized ABM campaigns for European buyers with CE compliance badges. Offer Declaration of Conformity as a lead magnet gated asset.
✅ ISO Certifications - International Standardization
Applicable To: Nearly all industrial sectors (equipment, electronics, chemicals, automation).
Popular Certifications: ISO 9001 (Quality Management) ISO 13485 (Medical Devices) ISO 22000 (Food Safety) ISO 14001 (Environmental Management)
ABM Implication: Position ISO as a competitive differentiator in technical content. Segment your ABM list based on ISO requirements by region or industry. Send pre-populated compliance checklists with sales outreach.
⚠️ RoHS / REACH - Environmental & Safety Compliance (EU)
Applicable To: Electronics, components, chemical inputs, semiconductors.
RoHS (Restriction of Hazardous Substances): Regulates use of toxic substances like lead, mercury, cadmium.
REACH (Registration, Evaluation, Authorisation, and Restriction of Chemicals): Focuses on chemical safety and supply chain disclosure.
ABM Implication: Include Material Safety Data Sheets (MSDS) in ABM assets. Target EHS (Environmental, Health & Safety) officers as influencers. Create secure portals for certification documentation access.
🏭 GMP - Good Manufacturing Practices
Applicable To: Pharma, biotech, nutraceuticals, and any industry producing ingestibles or injectables.
Key Focus Areas: Cleanroom environments, audit logs, training documentation, traceability.
ABM Implication: Educate buyers with ABM webinars featuring QA consultants. Share validated equipment use cases with GMP-compliant workflows.
Mapping Regulatory Stakeholders in Industrial Buying Committees
When selling into regulated industries, your ABM campaigns must go beyond engineers and procurement teams. Compliance is not a peripheral concern, it’s a deal breaker.
In most industrial manufacturing deals, compliance officers, regulatory heads, and quality assurance (QA/QC) teams act as gatekeepers or deal accelerators. Ignoring them is the fastest way to land in a procurement black hole.
Let’s break down who matters, what they care about, and how to influence them strategically.
🧠 Who are the Compliance Stakeholders?
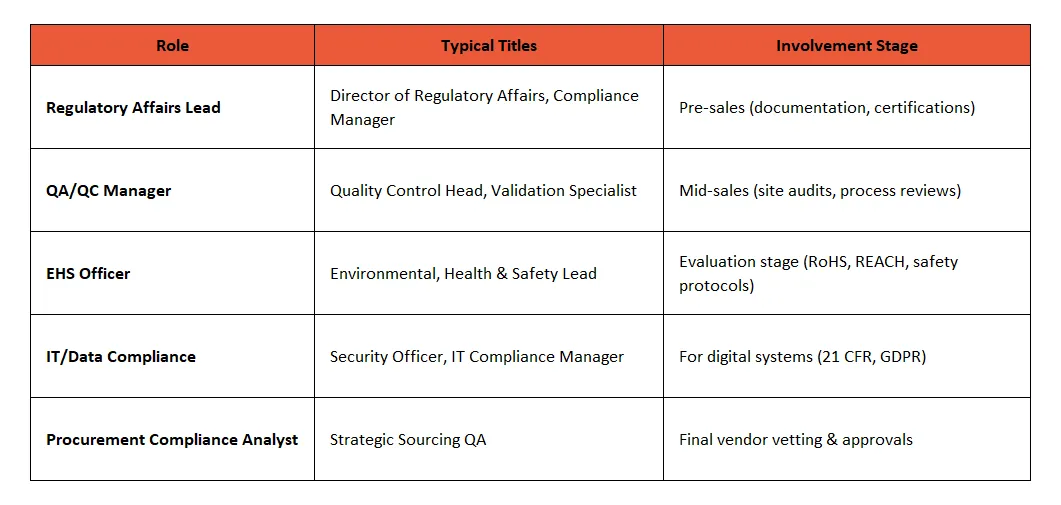
🎯 What do they Care about?
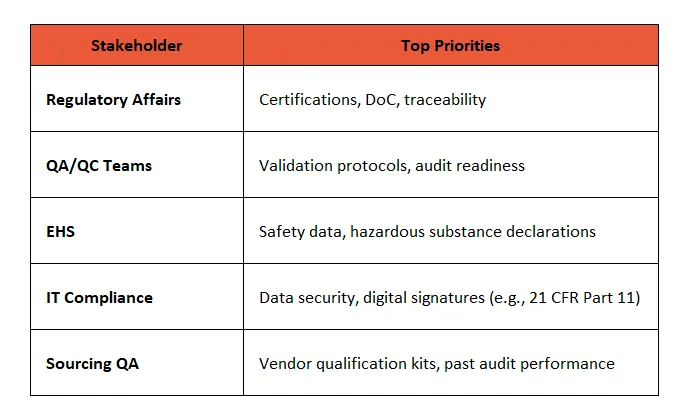
These roles are risk-averse by nature. Your messaging needs to be proof-heavy, process-focused, and documentation-first.
🧩 How to integrate Compliance Personas into ABM?
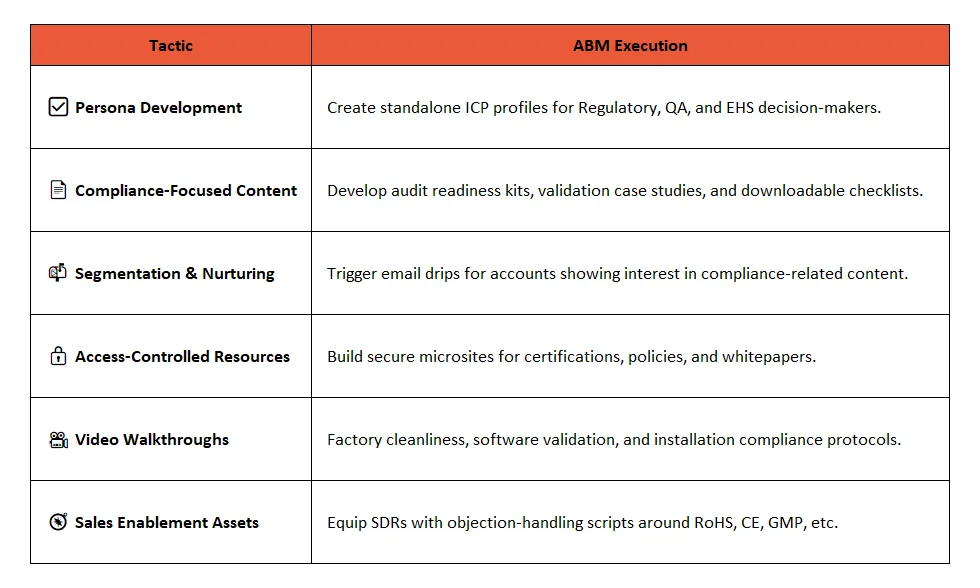
📍 ABM Use Case: QA Persona in a Food Equipment Deal
Let’s say you’re targeting a plant manager at a dairy processing facility. Your SDR lands a discovery call with Ops, but deal velocity stalls because the QA head hasn’t seen anything on cleanability, traceability, or compliance readiness.
Solution?
- Trigger a compliance nurture stream the moment the account shows Ops engagement.
- Send a “Sanitary Design Audit Pack” with cleaning cycle studies and regulatory references.
- Add the QA head to your CRM with a dedicated nurture campaign, including compliance alignment resources and audit-ready checklists / templates.
This is ABM at full maturity, cross-functional, stage-aligned, and strategically personalized.
Designing ABM Campaigns for Compliance Personas
Most ABM campaigns stop at “technical” and “economic” decision-makers. But in regulated industries, compliance personas are the silent veto-holders, and they don’t engage with sales decks or glossy brochures.
Instead, they respond to proof.
In this phase, we’ll walk you through how to build ABM campaigns specifically designed to engage, educate, and influence compliance stakeholders, including Regulatory Affairs, QA/QC, EHS, and IT Compliance Officers.
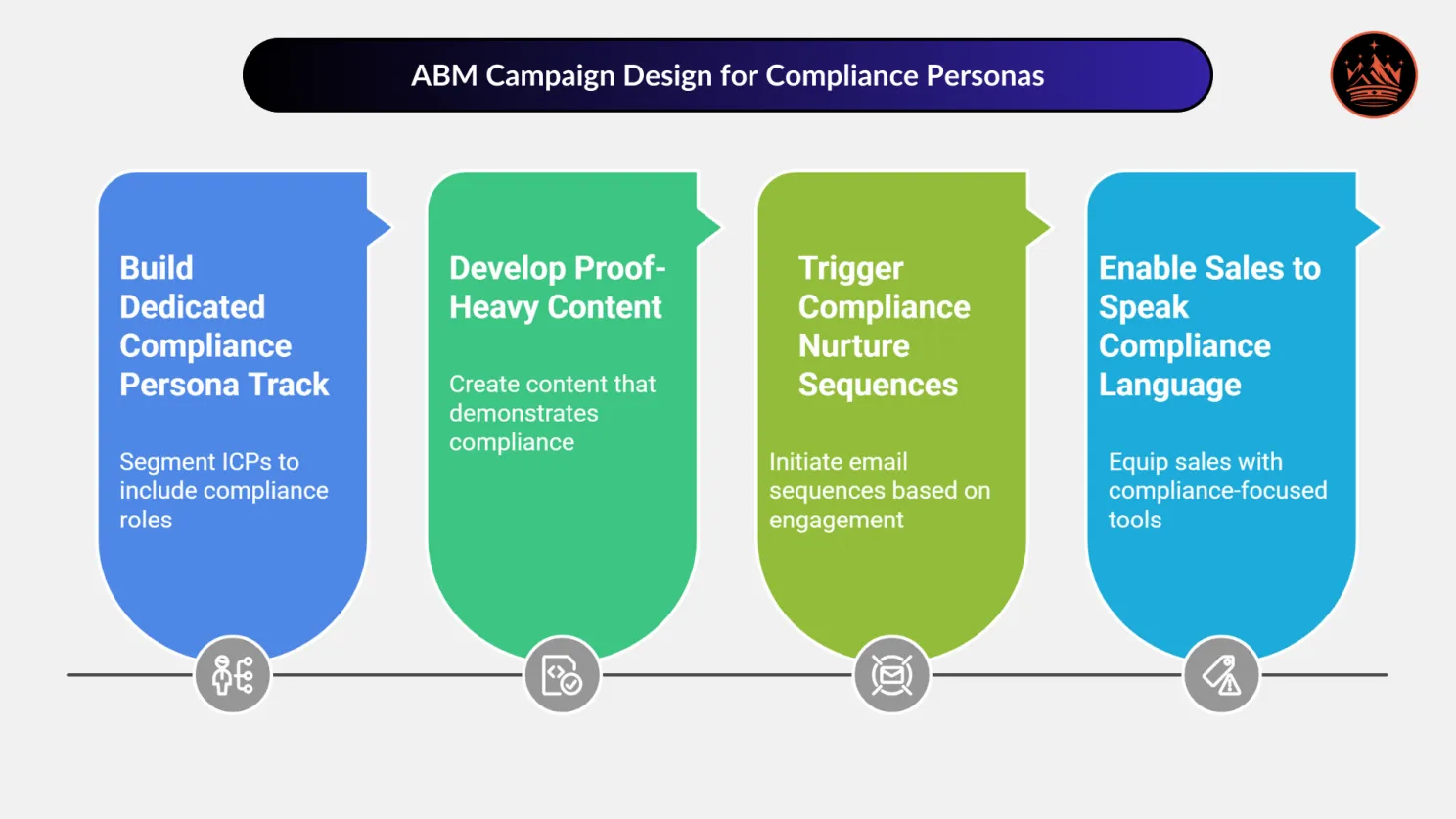
🎯 Step-by-Step ABM Campaign Design for Compliance Decision-Makers
✅ 1. Build a Dedicated Compliance Persona Track
- Segment your ICPs to include Regulatory Heads, QA/QC Leads, and EHS Managers.
- Map their unique pain points → Regulatory: Certification gaps, approval timelines; QA/QC: Process validation, audit failures; EHS: RoHS, REACH, emission control
Tip: Use historical deal data to identify common compliance bottlenecks. Your CRM will tell you where deals slow down, and it’s often right here.
📄 2. Develop Proof-Heavy, Risk-Reducing Content
Example: For pharma equipment, offer downloadable “Validation Protocol Templates” mapped to GMP and 21 CFR Part 11. That’s lead magnet for a QA Director.
📬 3. Trigger Compliance Nurture Sequences
- Track downloads and engagement from known compliance roles (via role-based email IDs or content metadata).
- Launch role-specific email sequences: Week 1: Case study on audit success; Week 2: Whitepaper on new regulation changes; Week 3: Invite to Q&A session with your compliance officer
Use VBOUT, HubSpot, or ActiveCampaign to set these up.
🤝 4. Enable Sales to Speak the Language of Compliance
Pro tip: Pair Sales and QA for an internal roleplay session. Let QA explain what their counterparts care about. Sales will never pitch the same again.
✳️ Compliance Personas are the Final Yes or No
In industrial sectors like food processing, pharma, chemicals, and electronics, if compliance teams say no, the deal is dead.
Your ABM must:
- Find them
- Speak to them
- Equip them
- Win them
Otherwise, your beautifully nurtured engineer or buyer will hit a wall you never saw coming.
ABM Compliance Content Assets in Action
In regulated industries, content isn’t just marketing, it’s evidence. The right content assets can accelerate deal velocity, bypass legal roadblocks, and create credibility with compliance teams before your Sales Engineer even books a meeting.
Let’s unpack the most effective compliance content assets used in ABM campaigns tailored for industrial engineering manufacturers.
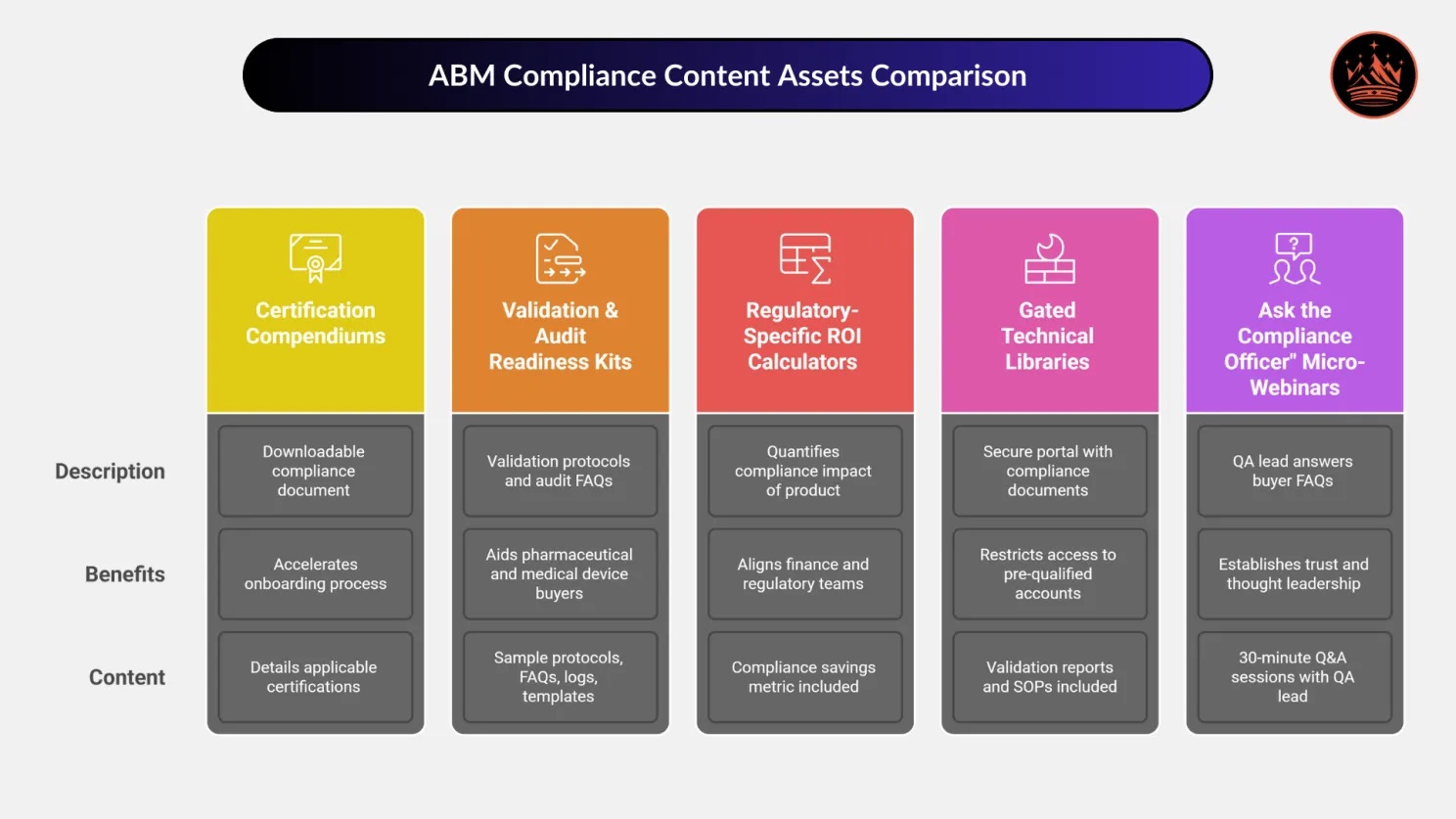
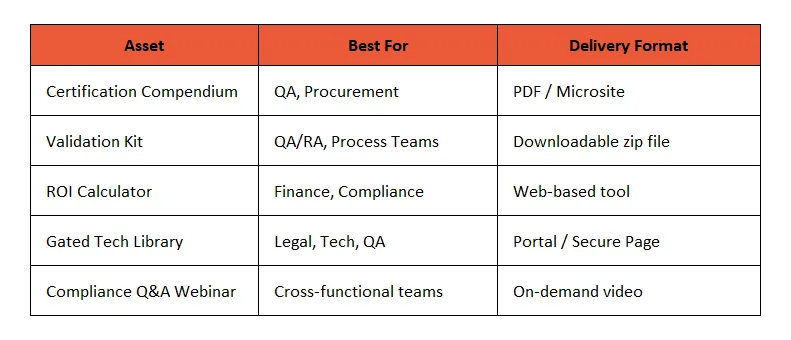
📂 1. Certification Compendiums
What It Is: A downloadable or interactive document detailing all applicable certifications (e.g., ISO 9001, ISO 13485, CE, FDA, RoHS, REACH, ATEX, etc.)
Why It Matters: QA and regulatory reviewers often delay vendor onboarding due to unclear compliance documentation.
Pro Tip: Break it into tabs by region (EU, US, APAC) or category (product, process, supply chain).
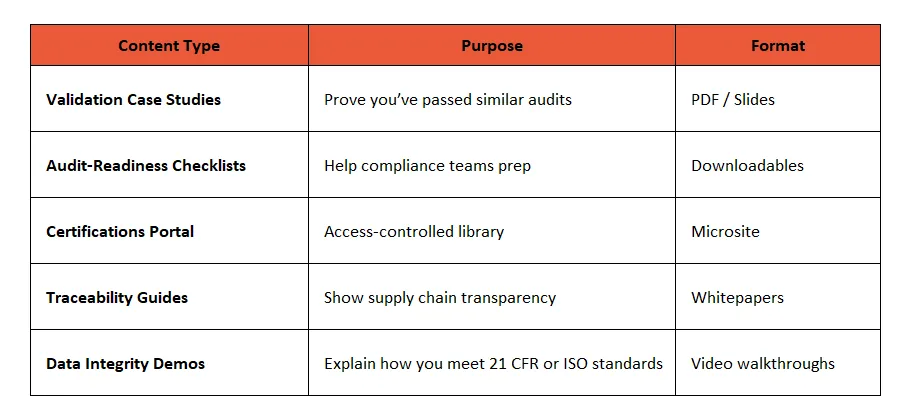
🧪 2. Validation & Audit Readiness Kits
What It Includes:
- Sample validation protocols (IQ, OQ, PQ)
- Audit FAQs answered by your internal compliance officer
- Equipment maintenance and calibration logs
- Change control processes and CAPA templates
Ideal For: Buyers in pharmaceuticals, medical devices, and food processing.
Example: A pharma filling equipment company offered downloadable FAT/SAT protocols and increased demo-to-deal conversions.
📊 3. Regulatory-Specific ROI Calculators
What They Do: Quantify the compliance impact of your product.
Use Cases:
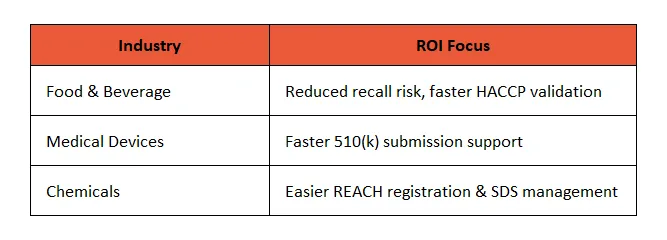
Pro Tip: Include a “compliance savings” metric alongside traditional ROI to get Finance and Regulatory in sync.
🧱 4. Gated Technical Libraries with Access Control
What It Contains:
- Validation reports
- Material compliance certifications
- Cleanroom compatibility checklists
- SOPs for equipment use in regulated environments
How It Works:
- Only available to pre-qualified accounts
- Access via secure portal (HubSpot, SharePoint, or custom integrations)
- Download notifications sent to sales for follow-up
🎓 5. “Ask the Compliance Officer” Micro-Webinars
Format: 30-minute sessions hosted by your internal QA lead answering FAQs from buyers.
Why It Works: Establishes trust and thought leadership with compliance personas who rarely engage with sales.
Bonus: Use questions submitted during registration to create future gated content assets.
✅ TL;DR → Content = Compliance Confidence
Aligning Sales, Marketing & Regulatory: Cross-Functional ABM Execution
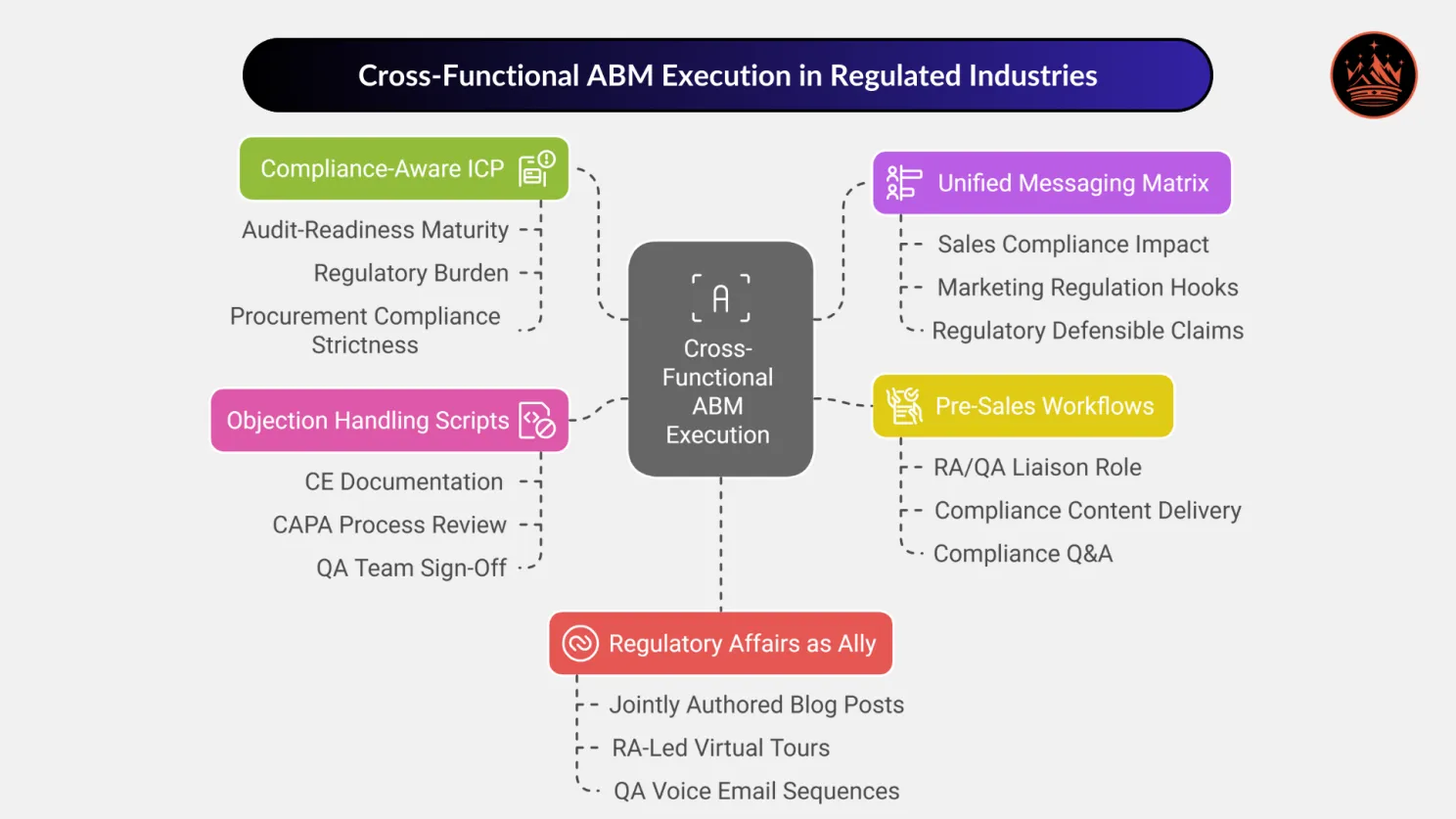
In highly regulated industries, siloed go-to-market teams are a liability. For ABM to succeed, Sales, Marketing, and Regulatory Affairs (RA/QA) must be tightly aligned, from messaging to objection handling.
Let’s break down the pillars of cross-functional collaboration that power compliance-safe ABM campaigns for industrial engineering manufacturers.
🧭 1. Build a Compliance-Aware Ideal Customer Profile (ICP)
Most ICP definitions focus on revenue potential, industry, geography, and pain points. That’s not enough in regulated sectors.
🔍 Add layers like:
- Audit-readiness maturity (FDA track record, ISO certification level)
- Regulatory burden (e.g., are they under EU MDR, cGMP, FSMA, etc.)
- Procurement compliance requirements (Do they require supplier validation programs?)
📌 Why it matters: Targeting a low-compliance-maturity account with technical assets that exceed their capacity can create friction, not trust.
🛠️ 2. Create a Unified Messaging Matrix Across Teams
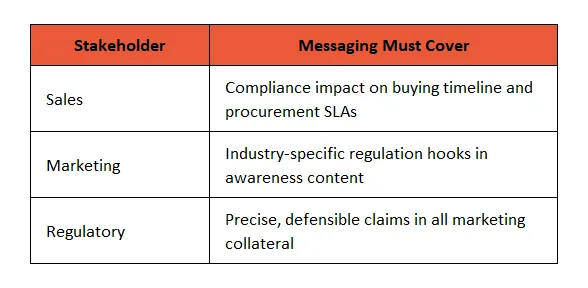
💡 Tip: Build shared messaging documents with regulatory-approved terminology, so Sales and Marketing don’t step into risky wordplay.
Example: Swap “FDA Approved” with “FDA Registered and compliant with 21 CFR Part 820.”
🤝 3. Design Pre-Sales Workflows That Include RA/QA
ABM campaigns often focus on marketing-qualified leads → sales conversations.
In regulated sectors, there’s a compliance qualification stage between Sales and Procurement.
Best Practices:
- Add a RA/QA Liaison role to account teams
- Trigger compliance content delivery post-demo
- Offer to schedule a compliance Q&A with your RA officer before contract finalization
📌 This shows maturity and professionalism, reducing deal friction.
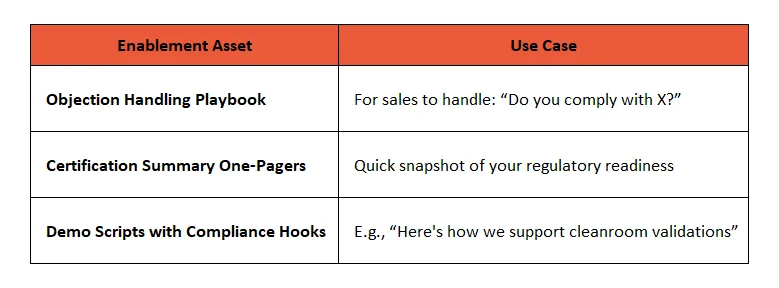
📣 4. Build Objection Handling Scripts for Compliance Pushback
Common friction points during B2B sales cycles in regulated industries:
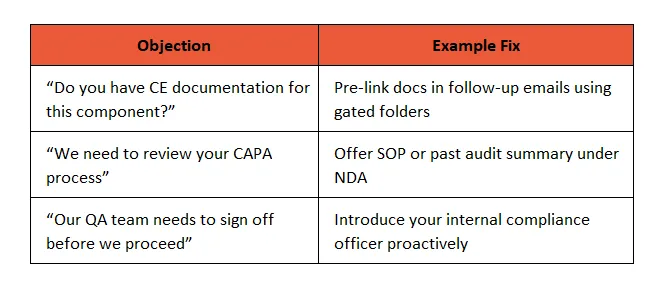
🧠 Enablement Tip: Build these scripts into LinkedIn outreach, automated follow-ups, and Sales Playbooks within CRMs.
🧬 5. Treat Regulatory Affairs as an ABM Ally, Not a Bottleneck
RA/QA professionals aren’t just compliance gatekeepers, they can co-create high-impact ABM assets when included early.
✔️ Examples:
- Jointly authored regulatory blog posts
- RA-led sessions during virtual factory tours
- Email sequences written from the voice of QA
This cross-functional alignment transforms ABM from lead gen into a deal acceleration engine, especially in complex, multi-stakeholder accounts.
Key Takeaways: Precision Wins in Compliance-Heavy ABM
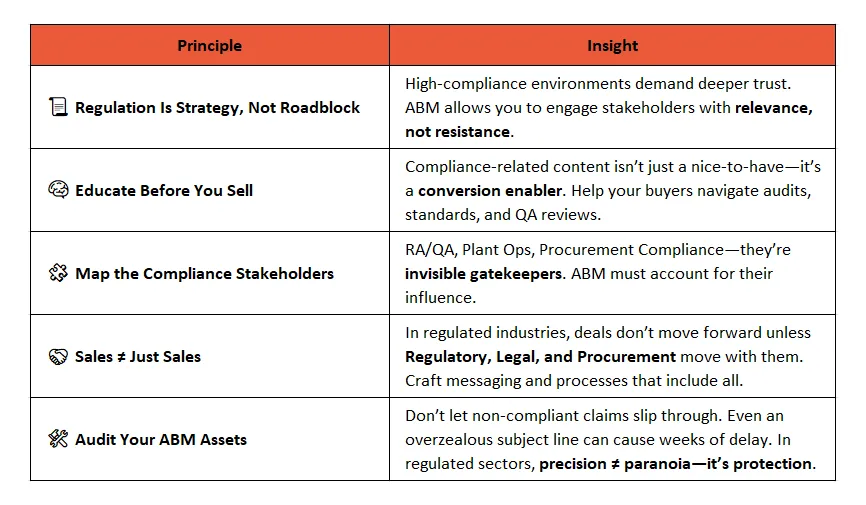
🔍 Final Thoughts → ABM That Wins in Regulated Markets Starts With Respect
Industrial sectors bound by FDA, CE, ISO, FSMA, RoHS, or cGMP frameworks don’t reward shortcuts. They reward trust, clarity, and consistency.
The most successful ABM teams in compliance-heavy industries aren’t just creative, they’re diligently informed. They speak the language of risk management, regulatory alignment, and stakeholder assurance.
Respect the process. Win the deal. Scale with confidence.